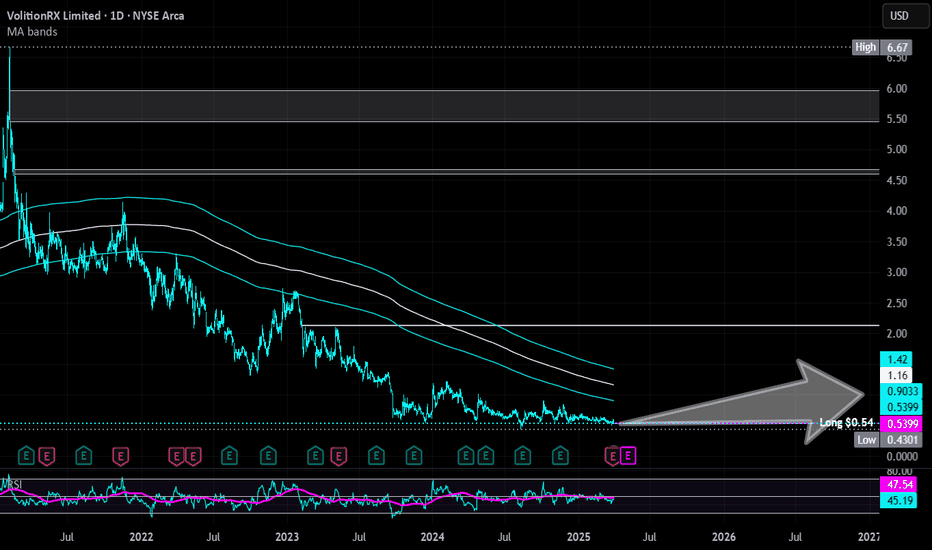
VolitionRX | VNRX | Long at $0.54***Stay away if you are risk averse (small cap with 300-400k daily volume and could go to $0).
VolitionRX AMEX:VNRX is a U.S.-based, multinational epigenetics company focused on developing blood tests for early disease detection, primarily targeting cancer and sepsis. Its Nu.Q blood tests are primarily for humans, focusing on early detection of diseases like cancer and sepsis. However, the company has also explored veterinary applications through its Nu.Q Vet product line, targeting cancer screening in animals, particularly dogs.
Recent insider purchases got my attention, with the CEO and Director each grabbing $100k worth at $0.55. Plus, many other insiders have recently been awarded options. The company is making progress in signing multiple licensing deals for their Nu.Q platform in the human market, with strong interest from large companies. Many development milestones have been made within their cancer testing program and more are likely to be announced. However, the company is unprofitable at this time, and this is a highly risky / speculative play. It may take years to unfold or be a total disaster and go to $0.00.
Rolling the dice at $0.54 with the goal to reach $0.75 and $1.00 in the coming 1-2 years. Analyst targets are in the $3.00-$3.50 range.
Cancer
AI Biologics firm Absci tie up with AstraZeneca on Cancer Drug Anglo-Swedish drugmaker AstraZeneca (AZN.L) has signed a deal worth up to $247 million with U.S. artificial intelligence (AI) biologics firm Absci (ABSI.O) to design an antibody to fight cancer, Absci said in a statement on Sunday.
Absci's collaboration with AstraZeneca aims for a zero-shot generative AI model designed to create new and improved antibody therapeutics, the company said. It did not say what kind of cancer they plan to target.
Absci applies generative artificial intelligence to design optimal drug candidates based on target affinity, safety, manufacturability and other traits.
Technical Analysist
Price Momentum
ABSI is trading in the middle of its 52-week range and above its 200-day simple moving average.
What does this mean?
Investors are still evaluating the share price, but the stock still appears to have some upward momentum. This is a positive sign for the stock's future value.
KVUE Split-off: A Response to JNJ's Cancer-Related Products?It is plausible to consider that Johnson & Johnson's decision to spin off Kenvue might be linked to an effort to mitigate potential legal liabilities stemming from its talc-based products, which have been implicated in cases of cancer in the US and Canada. This strategic maneuver could potentially offer a layer of legal protection. Shareholders are expected to transition from holding Johnson & Johnson shares to Kenvue shares in the upcoming week. A similar approach was attempted by MMM, although it did not yield successful results, causing a downward trajectory in its stock performance over the past two years.
From my perspective, the true rationale behind the division appears to be related to the numerous instances of individuals developing cancer due to Johnson & Johnson's talc-based products. A recent legal ruling mandated Johnson & Johnson to pay $18.8 million to a California resident who claimed to have contracted cancer from using its baby powder. This decision represents a setback for the company as it seeks resolution for thousands of comparable cases related to its talc-based products within a US bankruptcy court.
Johnson & Johnson recently disclosed detailed information regarding the much-anticipated division of its consumer healthcare venture, Kenvue. This move involves a separation of at least 80.1% of Kenvue shares, facilitated through an exchange offer presented to investors. Within this arrangement, shareholders have the flexibility to trade all, a portion, or none of their Johnson & Johnson shares for Kenvue stock.
The company is extending the choice to its investors, allowing them to opt for an exchange of shares for Kenvue stock. To incentivize this exchange, a 7% discount is being offered on the shares. However, there is an upper threshold of 8.0549 Kenvue shares for each Johnson & Johnson share. If this ceiling is not applicable, shareholders will receive approximately $107.53 worth of Kenvue shares for every $100 worth of Johnson & Johnson stock they intend to exchange. The execution of this exchange offer is anticipated to conclude by mid-August. Notably, this exchange program is voluntary and carries tax-free advantages.
Kenvue has outlined plans to distribute a portion of its available funds to shareholders through dividend payments. The company has recently initiated a quarterly dividend of $0.20 per share (equivalent to $0.80 annually), with the first payout scheduled for early September. This dividend framework translates to a dividend yield of 3.3% based on the prevailing stock price of approximately $24 per share. This yield marginally exceeds Johnson & Johnson's existing dividend yield of 2.8%.
It is likely that a significant portion of individuals holding Johnson & Johnson stock will opt to exchange their shares for Kenvue.
Based on this assessment, I anticipate that Kenvue's stock could potentially reach $30 in the near future.
Looking forward to read your opinion about it!
BriaCell Therapeutics Corp | Bria-MT™ | NASDAQ: BCTX TSX: BCTBriacell Therapeutics Corp has been trending higher on news - BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) #briacell #cancer #richtv #richtvlive #breastcancer (“BriaCell” or the “Company”), a clinical-stage biotechnology company specializing in targeted immunotherapies for cancer, is presenting positive clinical data from its lead product candidate, Bria-IMT™, summarized in four poster sessions during the 2023 American Association for Cancer Research (AACR) Annual Meeting held from April 14 – 19, 2023 at Orange County Convention Center, Orlando, Florida.
“Our data highlights the potential clinical value of the Bria-MT™ regimen in patients with advanced metastatic breast cancer after receiving multiple prior therapies,” said Carmen Calfa, M.D., of the Sylvester Comprehensive Cancer Center at the University of Miami, Associate Professor of Clinical Medicine, Principal Clinical Investigator, and co-author of the study of Bria-IMT™ in combination with PD-1 inhibitors pembrolizumab and retifanlimab. “These results are promising and the fact that patients have had a great quality of life thus far is remarkable. We are hopeful that this novel immunotherapy proves to be an effective therapy for our patients.”
“Our clinical findings continue to confirm our approach for our upcoming pivotal trial of Bria-IMT™ combination regimen,” commented Dr. William V. Williams, BriaCell’s President and CEO. “With over 40,000 annual deaths in the U.S. alone, advanced metastatic breast cancer remains an unmet medical need. Patients who have only months to live tend to avoid current therapies that are proven ineffective and are associated with excessive toxicities. BriaCell’s regimen has shown robust clinical efficacy, better than expected survival outcomes, and an excellent safety profile in this very difficult to treat patient cohort. We are seeing benefits in patients who failed other treatments and/or cannot tolerate the harsh side effects of other therapies.”
Roche Holding AG bullish scenario:The technical figure Triangle can be found in the daily chart in the German company F. Hoffmann-La Roche AG (ROG.vx). Roche is a Swiss multinational healthcare company that operates worldwide under two divisions: Pharmaceuticals and Diagnostics. Its holding company, Roche Holding AG, has shares listed on the SIX Swiss Exchange. Roche is the fifth largest pharmaceutical company in the world by revenue and the leading provider of cancer treatments globally. The Triangle broke through the resistance line on 07/09/2022, if the price holds above this level, you can have a possible bullish price movement with a forecast for the next 10 days towards 333.65 CHF. Your stop-loss order, according to experts, should be placed at 311.75 CHF if you decide to enter this position.
Roche announced the launch of the Digital LightCycler System, Roche’s first digital polymerase chain reaction (PCR) system. This next-generation system detects disease and is designed to accurately quantify trace amounts of specific DNA and RNA targets not typically detectable by conventional PCR methods.
The Digital LightCycler System will allow clinical researchers to divide DNA and RNA from an already extracted clinical sample into as many as 100,000 microscopic individual reactions. The system can then perform PCR and produce highly sophisticated data analysis on the results.
Risk Disclosure: Trading Foreign Exchange (Forex) and Contracts of Difference (CFD's) carries a high level of risk. By registering and signing up, any client affirms their understanding of their own personal accountability for all transactions performed within their account and recognizes the risks associated with trading on such markets and on such sites. Furthermore, one understands that the company carries zero influence over transactions, markets, and trading signals, therefore, cannot be held liable nor guarantee any profits or losses.
BioNTech showing a solid entryBNTX is finally concluding a year long descending triangle pattern. I see a low risk entry at $150 and will be buying. Stop loss set at $138.49, risking about 8.5%. If we see a large move upward, I could see the stock retesting both its 200 day moving average (likely at $183) and its next major resistance after that at $295. A potential of nearly 100% profit (not to say its a sure bet or smart to dump huge amounts into the trade without the stop loss...). Fundamentally, the company has a P/E of 3, no debt, and a huge free cash flow to fund its mrna platforms for a lot of other diseases partnering with some of the largest pharma companies in the world. Yet another variant of covid, upward pressure from the 50and100 day MAs, large portions of the world unvaccinated, and president biden contracting the virus could be potential catalysts for a move. If you're looking for a bio tech exposure this is a stock that seems to be set up well. Good luck to all.
$BCTX poised to bounce $BCTX - BriaCell is a cancer treatment biotech that recently dropped 40% AFTER receiving fast track designation for FDA approval. The drop was somewhat coincidental as it's month long bull run finished with a FinTwit pump and dump. BUT the retrace is in and it's approaching historical trend lines and levels of support and looks poised to bounce in the coming week. TD9 is flashing a buy signal, but I wouldn't be shocked if it begins to curl throughout the week. Looking to enter a long swing trade to ride it back to previous highs.
GOVX Corporate Update soon | 10X Upside Potential Analyst RatingGOVX GeoVax Labs is a biotechnology company developing immunotherapies and vaccines against infectious diseases and cancer.
GeoVax Labs will report Q1 2022 financial results on April 27.
Jason McCarthy from Maxim Group has a $10.00 price target for GOVX.
The stock is now $1.01.
Market Cap of only 7.23Mil.
This is a 4X upside potential short term stock in my opinion.
CWBR Upside PotentialCWBR closed 2 months ago a $15.0 Million Public Offering of Common Stock and Warrants at $0.72, so this is a safe upside in my opinion if you want to enter now.
On the other hand, Cantor Fitzgerald brokerage has a $2.5 price target for it.
52 Week Range 0.34 - 2.27 Just bounced from its lowest support.
SESN Price TargetOn 8/17/2021 Canaccord Genuity brokerage Lower Price Target for SESN giving a Buy rating from $7.00 to $3.00.
Sesen Bio (SESN) flagship drug candidate Vicineum, failed to obtain FDA approval, but they will try a second time to get the regulatory go-ahead for the bladder cancer treatment.
buying long term calls could be a strategy for this one. volatility is likely after they announce the second FDA submission.
GRTX important Price Target Upgrades Today !An "error" in previously released results sent Galera Therapeutics, Inc. (GRTX) stock price down by more than 70% in October.
Today GRTX was upgraded today by HC Wainwright to buy, 10usd price target and by BTIG Research to 15usd price target.
Have you bought it yesterday?
isr bREAKING OUTMy kid came by and when i was looking at the chart, she said: hey look! a bird! and at the time, there was no bird there was only a small cone I drew for the pennant at the tip of the retracement.
I zoom out a little, and noticed, wow you are right the bouncing kind of looks like ahead when you add an eye when I zoomed out more, the chart had a real recollection so I drew it. I think this chart is bullish and this tells tales about the physical element and visual elements of stocks
they need to look good, and they project a feeling of motion to people's psyche.
the shape, is soothing, smooth, looks organic, (even tho it isn't it is highly manufactured by the shorts)
just thought it was interesting to share...
I think this stock will go to 8-10$ despite what lots of people are saying in the Weibull chats.
This is good lung cancer treatment stock and I think people would be wise to have some. it had a nice-sized green hammer this morning.
CVM and Multikine; Rating and Confidence ReaffirmedDisclaimer
I am long $CVM to the tune of X0,000 shares (and growing). I genuinely believe, as a scientist, biochemist, and analyst, in Cel-Sci Corporation's Multikine. This article serves as the thesis that $CVM deserves that I have given to other stocks. As always buy shares, options are dangerous and are often used to manipulate the stock.
Now that we have gotten through that:
This is in no way, shape or form, fluid and function, an analytical, qualitative or intelligent compte rendu. There is absolutely no financial advice here because the only financial advice I can give is to research, research, and research. The purpose of this analysis is to serve as an example of an investigation into a company's background, fundamentals, and assets through various lenses to determine if it is a good potential investment for you. The function of this write up is to serve as an educational resource for investors looking to understand how to find good investments. So read and learn some things about a company that cured cancer, yeah, the big C.
Thesis
It is upon rare occasion that one must look towards the past for moving into the future. Then certainly we are led here, a company starting it's preclinical scientific journey in the 1980s. A relic of a flare in a field few thought held value, immunology. Now, it is one of the fastest growing fields in medicine and science, where immunotherapies are quickly becoming the next generation of cancer treatments. Where others have come, and shown promise, Multikine has been here, for decades, toiling in the dark, fighting off bankruptcy, manipulative media and share attacks, and snobby scientists and doctors. Cel Sci Corp stand on victory, their quest coming to an end, and the market coming to the beginning of their own: How much is a drug that safely cures cancer worth?
Keytruda, itself an inhibitor of the immunoblockade, brings in nearly $15 billion in revenue per year over the last 3, with some estimates that this is early rather than late. Keytruda's therapeutic effects limited to an average of 6 to 8 months of survival time depending on cancer, Immunotherapies quickly became the sensation despite "limited" clinical benefits. Molecularly speaking- that is under the hood- Keytruda's true glory served as a limited toxic drug. Standard chemotherapeutics rely on a simple principle: do as much damage to quickly dividing cells, as much so to cancerous cells. This damage is not specific to the cancer cells: normal, happy, healthy cells get damaged too, often leading to them dying. But sometimes they don't die, they carry that damage on and then become a cancer cell later on. Sometimes that which doesn't kill you, makes you stronger, and sometimes it makes something inside you stronger that then kills you. Cisplatin, itself a platinum based drug that binds to the DNA, RNA and proteins in a cell causing them to clump up, get degraded, fixed if possible, leading to massive chromosomal damage (the genetic code). Normal cells take up the Cisplatin to a similar degree to the cancer cells (in fact cancer cells can make more of a special drug pump, then pump all of the drug out of the cancer cell to be consumed by the healthy cells, thus helping it grow), except normal cells have special checkpoints in place to look out for damaged DNA to fix it, or if unfixable, destroy it. 99.9% of the time, the damage gets fixed, or the cell dies, and 99.9% of those times where the cell continues on and becomes cancerous, the immune system finds it and kills it as it identifies it shouldn't be there. Cisplatin has an extremely high rate of cancer reoccurrence, keeping patients alive through cancer round 1, but often failing in round 2 or 3, but also being the cause of round 2 and 3.
Cel-Sci Corporation's Multikine, a mix of interleukins and cytokines used to communicate between the working immune system that an issue is there and needs to be addressed, showed excellent promise in Phase 1 and 2 clinical trials. However, clinical trials have been difficult and slow for a multitude of reasons; the immune system is extremely complex, and until recently there was no technology available to easily study the system in the true depth necessary. Pre-clinical research could not be done on standard laboratory animals as their immune systems are nearly completely defective. From there, a biochemical understanding of the nature of treatment itself impossible until recently, clinicians had little to no idea on what therapeutic response should look like, or what it could look like. Perhaps in some medieval notion, doctors might have considered activating the immune system to fight cancer would be similar to fighting a virus; shakes, shivers, fevers, sickness. When none of these effects, or even no side effects occurred, perhaps there was a considerable amount of white coats in large rooms shaking their heads assuming the project dead.
Through nearly 10 years of a Phase 3 in Head and Neck cancer, starting with a criminal and negligent CRO leading to a successful trial and large monetary recuperation, and ending with a stock-collapsing headline of " 14% increased 5 year survival over Surgery and Radiation alone, less than 10% increased 5 year survival over Surgery, Radiation and Cisplatin ". Perhaps in some small way, Cel-Sci Corporation never had a chance of an easy time, starting from the uphill battle through Ivy Tower elite scientists, ego-heavy medical doctors without a degree in immunology, manipulative short sellers and evil hedge funds reminiscent of the vile Michael Milken; through 3+ decades of pushing the entire medical field forward, paving the way through doctrine and dogma, leading to a breakthrough therapy with no significant side effects through hundreds of people, and an impressive long term survival rate in a cancer that has had no medical breakthroughs in just as long.
Previous articles by this author and others have suggested that the Cisplatin arm of head and neck cancer patients is likely at or below 62%, which is Multikine's 5 year survival topline result. However, the numbers drop from there, and the specifics of that population look even worse. Cisplatin has an extremely low disease free survival/cured rate (where cure is defined as 5 years without cancer), and high secondary tumour incidence. That isn't to say that if given a choice between death and Cisplatin by the oncologist, the patient should absolutely chose Cisplatin, but very soon, they won't have to. Oncologist's will be able to choose a treatment profile in waves, where the first wave is safe therapies, specifically those with low death/suffering rate, such as surgery, radiation, and perhaps a side effect free drug that activates the immune system leading to a long term immune response capable of suppressing the immuno-evasion of the tumour cells right through to 5+ years of healthy life. Patient's from the Phase 2 trial of Multikine had a significant shrinkage of tumour, composite loss of pain, regained freedom of movement in tongue, etc; the drug was an absolute success. In fact the muted response from the clinical results from the trial paper is astounding. While the world must wait for the Phase 3 results to be published in a peer-reviewed journal, a critical step in validating and diffusing the data, the door to the FDA is coming closer and closer. Within the next several weeks or months, a pre-BLA meeting is expected between the FDA and CVM, where they will discuss the next steps and review keynotes of the data organizing it for the final submission and drug review process through the hands of dozens of scientists and medical professionals. Here, the FDA will see as any scientific or medical eye will find, Multikine is a breakthrough drug that will revolutionize cancer treatments.
Emulating Keytruda's path from rich to uber-rich, Multikine will seek for, and gain, approval for Head and Neck cancer with an open label allowance for other indicated cancers. Keytruda went from seeking approval, to nearly complete market approval in a matter of 6 months. While Cel-Sci Corporation does not have the wealth and power to force such a keystone move on the FDA, following historical precedent and medical need, Multikine will be in cancer patients across the spectrum as long as they have a working immune system by the end of 2022, just as Keytruda was in the same position at the same time scale. There is nothing exclamatory or insane about this statement, it has been this way for every disease and every drug that has gone through the FDA; a standard of care exists, experimental drugs better than SOC come along, the new SOC is formed through years of main-stream drug use showing wide market agreement with the clinical trials. Even in Keytruda's own question, failure of all primary or secondary endpoints has not stopped it from getting added to the therapeutic regiment for nearly every cancer, and ultimate approval for said cancer by the FDA.
Multikine's lack of additional clinical response with Cisplatin was a scientific inevitability. Cisplatin damages fast growing cells, the immune system being one of the fastest. Cisplatin often leaves patient's immune system naïve, leading to a need for a geriatric round of childhood vaccines! However, the scientific team at Cel-Sci realized this pre-Phase 3, leading to the establishment of the clinical trial arm without Cisplatin. While bear's might call this part out as evidence of CVM's failure, it is specifically opposite. Cel-Sci Corporation's leadership strong-armed the FDA to allow this branch of the trial, and because of it, have illustrated multiple paramount issues;
Immunotherapies take a long time to work in cancer, but they do work.
Immunotherapies do not work with mainstream chemotherapeutics that have the potential to inhibit and mute their effects.
Cisplatin is a bad drug that kills people and is only used because sometimes it kills the cancer before killing the person.
In this thread, CVM's true long term worth comes in the clinical lessons learned, not just the lives saved. And Multikine does save lives. If only indicated for the current population, head and neck cancer without cisplatin treatment, an estimated 12,000 people a year will be saved by getting Multikine too. Financially speaking, that is a lot of dough. If given to all available patients in the 144,000 group and sitting at Keytruda costs per year of $150k, Multikine could see revenues of $21.6 billion within 3 years. Any deviation in patient population upwards, as is expected, could lead to the most economically impressive drug of all time, and a metric %&#@ of lives saved.
While the current price action is little more than the chaotic meandering of algorithms, market makers, and maleficent hedge funds trying to prevent a massive squeeze from decades of phantom shares being sold, the inevitable trajectory is upwards. This author will leave the curious investor's mind to wander through previous articles and historical acquisition deals of similar scope, but there has never been a drug with more active therapeutic potential than Multikine. When collective cancer research was losing it's mind over Keytruda/PD-L1/PD-1/CAR-T cells, etc, there was only pre-clinical evidence and all early clinical evidence was looking rough. Multikine is out of Phase 3s with a perfect safety record, a massive amount of therapeutic evidence showing a massive clinical benefit, 14% 5 year survival rate in Head and Neck cancer patients leading to a similar short term, and much better longer (>5yrs) term survival rates than Cisplatin. While the catalysts are in full scope for $CVM from today's $11, to a potential $X00, the most important element is this:
Fuck cancer.
Trading Beta
TradingView will enjoy my use of the term Beta with their new rock climbing theme.
The chart shows current price valuations across bear, bull and base case estimates.
From a basic standpoint, this author continues to buy shares, especially as the price continues to be pummeled by the banks and large hedge funds offloading their puts and getting in more calls. Ultimately, a short squeeze/retail interest/increased volume should yield a $50-75 bear price, where appropriate dissemination and understanding of the clinical data and therapeutic implications could lead to an initial bull run to $150-200 giving the company a market capitalization around $5-7 billion (a measly sum considering $OCGN's worth given vaccine uncertainty and $SAVA with pre-clinical data issues and a drug early on in a lengthy and competitive clinical process).
Reasonably speaking, the goal is to amass a large sum of shares, never sell, use leverage and margin to build up secondary positions as $CVM's long term success is guaranteed. Current and future short sellers will force smart moves, but appropriate risk management practices leave this as the primary goal.
Secondarily, amassing large quantities of shares and selling monthly covered calls at critical price points as a way to create sustaining, secondary income and a forward momentum/pressure on the stock promises some level of efficacy.
Tertiarily, options around impulse waves while keeping my own position stable and growing yields more favourable derivative outcomes. Cel-Sci Corporation no longer needs to be acquired to achieve maximum market capitalization, and in some ways, may best achieve shareholder value by utilizing their new manufacturing space to continue on their smaller scale, guaranteeing current investor access to a double digit billions in yearly revenue within 3-5 years.
Links to Previous articles
Links to other highly qualified articles
There are many more articles than this, I just tried to choose a few from the various mixed media sources. I absolutely encourage any investor to see what others have written on the matter, but do not, under any circumstance, believe any single person, including me, without doing your own research and confirming or denying any and every thing. It is absolutely imperative to double check, triple check any and everything.
www.cvmresearch.com
seekingalpha.com
www.sciencetimes.com
seekingalpha.com
finance.yahoo.com
biotechhealthx.com
Disclaimer
Thank you for reading, please review all links, articles, press briefings and scientific data. If ever any questions, please comment, message me, or find me on twitter.
SESN Sesen Bio Selloff | Buy the dip???SESN received a preapproval for the dug, Vicineum, but not in its present form. and the market overreacted to it. sold at market prices. and the price went down to 0.86usd, its strongest support.
The FDA has determined that it cannot approve the BLA for Vicineum in its present form and has provided recommendations specific to additional clinical/statistical data and analyses in addition to Chemistry, Manufacturing and Controls (CMC) issues pertaining to a recent pre-approval inspection and product quality. (businesswire.com)
And they don`t have only one product in their pipeline.
Sesen Bio focuses on designing, engineering, developing, and commercializing targeted fusion protein therapeutics (TFPTs) for the treatment patients with cancer. The company's lead product candidates include Vicinium, a locally-administered targeted fusion protein that is in Phase III clinical trials for the treatment of BCG-unresponsive non-muscle invasive bladder cancer (NMIBC); and VB6-845d, a product candidate for use in the treatment of various types of an anti-epithelial cell adhesion molecule (EpCAM)-positive solid tumors. It also develops Vicinium in combination with Durvalumab, which is in Phase I clinical trials for use in the treatment of BCG-unresponsive NMIBC; and Vicinium in combination with AstraZeneca's checkpoint inhibitor for the treatment of squamous cell carcinoma of the head and neck. Sesen Bio, Inc. has an agreement with Leiden University Medical Center to co-develop an imaging agent. (marketbeat.com)
On 8/11/2021 BlackRock Inc. reported 10,657,812 for a total of $49.24M +264.7% increase and an ownership in SESN of 6.150%
On 8/13/2021 Vanguard Group Inc. reported 8,503,982 for a total of $39.29M +18.2% increase and an ownership in SESN 4.339%
My price target is between 2.7 to 3.2usd.
I look forward to read your opinion
BYSI BeyondSpring Price TargetBYSI BeyondSpring drug, plinabulin, increased overall survival and improved other measures of disease while staving off a dangerous side effect associated with Chemotherapy.
BeyondSpring is preparing to ask for plinabulin approval in the U.S. and China.
On 8/4/2021 J. Pantginis from HC Wainwright brokerage Upgraded BYSI BeyondSpring from Neutral to Buy giving a Price Target of $100.00
My price target is the all time high resistance of 48usd until we hear some news from the FDA.
CVM; Breakout from bear pennant, prime for liftoffDisclaimer
General disclaimer here.
Short but sweet, we have sustained breakout from the bear pennant, we have a dwindling short pool (460k as of today borrowable from Fidelity, down from ~600k on Monday).
Less kick in the shorts, and they have until the 20th of this month to sustain the price channel and try to get it under 7.5.
Meanwhile, more and more groups are buying up shares in small amounts, likely on the back of bigger entities buying up, as State Street increased their position on the drop from $25.
We are only a few weeks away from a catalyst with the pre-BLA meeting, pushed by more and more FDA breakthrough news.
This is a great time to look for an entry on shares.
If you buy options, a good rule I use is 90-10. Only use 10% of funds on the options, that way if they go south, you are still likely to end up green on the overall trade.
With that said, we have no ceiling as the drop from 26 was abberative manipulation, we have a gamma squeeze lining up from $9 and into the $20s. Theoretical M&A prices are obscenely high, even conservative values at $5 billion would give this a x15 multiple.
Have fun y'all, always be safe, and please do your own research to verify. And always ask questions!
whalewisdom.com
CVM; A Win for Medicine, a Death Sentence for Cancer and shortsDISCLAIMER
I am financially invested in this stock, I have 4200 shares, and a considerable amount of calls for an unspecified date and amount. So yes, I, in a very big way, am selling you. I financially benefit if you, the investigative reader, buy CVM shares or, very possibly, calls. That means that this is not financial advice, this is a sales pitch, so if you don't think you can separate pitch from information, avoid this article. I have been apprehensive on writing this article as I accept my bias, and while I will present the following analysis of the study results, and possible implications, I am extremely biased and my ability to make a rational decision could 100% be compromised. What I present is my best explanation of the trial results, what I believe to be occurring to the price, and some of the scenarios Multikine could play out.
Now that we have gotten through that; This is in no way, shape or form, fluid and function, an analytical, qualitative or intelligent compte rendu. There is absolutely no financial advice here because the only financial advice I can give is to research, research, and research. The purpose of this analysis is to serve as an example of an investigation into a company's background, fundamentals, and assets through various lenses to determine if it is a good potential investment for you. The function of this write up is to serve as an educational resource for investors looking to understand how to find good investments. So read and learn some things about a company that cured cancer, yeah, the big C.
Thesis -Clinical Analysis
www.businesswire.com
I made this very basic figure: twitter.com
Big question is which treatment would you ask for?
The hard line is a 14% increased 5 year survival in Head and Neck cancer patients with a solid tumour being treated with Multikine as opposed to the current standard of care(SOC). All without a single safety issue, meaning it wasn't making patients sick, and as it was given 3 weeks before surgery and radiation, there was plenty of time for the immune system to go into hyperdrive mode and present serious issues.
I know I write a metric $#%@ ton of words, but y'all, I try and always let the data do the work. This is as pretty as it gets in my shoes. At the end of the day, there are no sub-statistics that matter, because in every single patient population that got Multikine, there was an increase in survival. CIZ is a weird little multivitamin that has been found to boost a ton of drugs' effects in the clinic for completely unknown reasons, or at least they were unknown last time I checked, but it is more designed than developed, but it legitimately does some incredible things. My hypothesis is that the mix helps reduce oxidative stress, which isn't a major issue for normal people walking around, which is why it is never found to have an effect on its own in any clinical setting, but it does seem to help in these various trials. This is a little too scientific, but oxidative stress is turning out to be the number one pathway in disease and just general cellular maintenance. I actively work on this scientifically, so there is a lot of contentious hypothesizing, but I am finding that oxidative stress would have been a relatively simple evolutionary step to take early on in macro-cellular organisms, but humans have done everything except. One of the major issues that is rarely thought of in a scientific lab setting is the oxidative stress of the environment caused by the massive amount of cellular death from therapeutic death of cancer cells. Sometimes killing the cancer too fast can overload the bodies ability to dispose of the dead cells, and cause major systemic issues and death. Yeah, sometimes killing cancer can just kill the patient because they are too weak to handle getting rid of all the dead tissue in them. No, this is not an easy task, but damn if we don’t keep trying. Also oxidative stress and inflammation are very linked!
Cisplatin is a toxic drug, meaning it is bad for you. The only reason you take it is if there is something worse for you that it might kill first. This is one of those situations where my background as a biochemist and cancer research gives me a leg up on the average investor, I have personally used cisplatin in experiments on primary human cells testing cancer-genesis with various DNA damaging agents. While I can't give those results to you, I can summarize it as yeah Cisplatin is pretty freaking awful, and it should only ever be used if there is nothing else.
Enter the reasoning for going after Head and Neck cancer; 6% of all cancer diagnoses per year (meaning big bucks) + no new treatment approved in over 2 decades + orphan drug designation from FDA making it a little easier to file the paperwork. This point needs to be emphasized hard here, there is nothing unique to head and neck cancer that makes Multikine be specific to it. CVM don't intend on applying for FDA approval for a small subset of Head and Neck cancer patients and biting the bullet on a smaller population pool. CVM are going after FDA approval for that small pool with immediate open label use. I will testify before a court of law, I would formally request Multikine as treatment if I were diagnosed with cancer tomorrow. I am very, very, very excited for Multikine to get to the clinic, this is the game changer that we have been waiting for and it is killing me that we are not celebrating this one. Fuck the stock price, can we get a round of fucking beers for a god damn cure for cancer.
This is the only important line from the release:
In addition, as the OS results for the lower risk of recurrence patients (no chemotherapy) are significant (two-sided p=0.0236, HR=0.68) and the effect is robust, durable and increasing over time, CEL-SCI plans to seek FDA approval for Multikine cancer immunotherapy in this underserved patient population.
OS
-overall survival- overall survival benefit being increased percent of people who survived from Multikine- percent more living people thanks to Multikine
lower risk of recurrence patient
- patients with a tumour grade of Head and Neck cancer deemed to be low risk of metastasis and coming back post-therapy
significant two-sided p=0.0236
-essentially boils down to could only happen by chance once in 42.4 times, meaning the difference in survival in the Multikine population is in fact, not simple chance, but actual clinical correlation/causation.
HR=.68
-HR means Hazard Ratio, is a direct ratio of probably of death. A HR of .68 means that for every singular patient who dies from the Surgery+Radiation arm, only .68 people die from the Multikine+Surgery+Radiation arm. Lower the HR, better the outcome
The effect is robust, durable and increasing over time
-I wouldn't know what that meant without them explaining that the overall survival advantage increases past 5 years. Where cured of cancer is defined as 5 years without remittance, Multikine is curing cancer. Of course this is hearsay until we see the statistic results ourselves, but I would hesitate doubting a company that could be crushed if they didn't back that up with real numbers.
Now, the bigger picture of the trial:
twitter.com
To put it bluntly, Multikine and Cisplatin don't work well together. As of right now, we have no idea if Cisplatin + Multikine has an overall survival 5 year rate of 9.5%, or an overall survival 5 year rate of -100%. My personal theory is that Cisplatin is going to destroy any effect that Multikine had on the system. Multikine is injected into the primary and secondary tumour sites 3 weeks pre-surgery followed by cisplatin and radiation or just radiation. Multikine has 3 weeks to do its job before surgery comes in, followed by radiation and Cisplatin. If Multikine didn't complete its job in 3 weeks 100%, as in there was any considerable amount of cancerous tissue remaining, Cisplatin is going to come in and do it's job, like it or not.
Cisplatin is like Chris Farley in a Little Coat to those tumour cells. To the few that manage to survive and make the EMT transition, or if the cancer stem cells survived (if those are even real, my guess is that this is a pool of cancer cells with an extremely fluid transcription profile that are kind of going back and forth between EMT-MET), cisplatin is ripping through their genome like a coffee bean grinder. I thought long and hard about the appropriate level of destruction I have seen from chromosomal spreads of cells on cisplatin, and coffee grinder feels pretty damn close sometimes. Cisplatin works by sticking to parts of the DNA in multiple places, making it impossible for the cell to make an exact replica of its genome, thus inducing mutations that could lead to death. Cisplatin causes more damage in quicker dividing cells; Cisplatin causes DNA damage, DNA damage that will be ignored and lead to fatal mitotic chromosomal splits/rearrangements, or DNA damage that will be addressed and fixed by healthy cells unless it is too great in which case they will choose to die rather than risk becoming cancerous later on.
Turns out some cells are just more selfish than others, they don't want to die. Cisplatin cancer reoccurrence is a very real thing that has been suppressed for a long time for a laundry list of reasons, namely being doctors and clinical scientists genuinely don't believe the patient is intelligent or informed enough to decide their own treatment course. That is putting it harshly, but essentially, Cisplatin was approved a long time ago and there are millions of people who have gotten millions of collective years on this planet thanks to Cisplatin. However, we are at the point where serious clinical work is being dragged down by Cisplatin because of the need to put every patient on it that can be on it regardless of theoretical alternatives. That and fighting with the FDA is pointless, they can just walk away and tell you go screw yourself. If CVM had any intention of having an arm from the get go of the total patient pool being only Multikine + Surgery + Radiation, I am 100% positive that 5 minutes talking with the most monotonous man in an off-white shirt with a terrible chemical structure tie, an FDA badge on a lanyard around their neck, sitting across from them as they awkwardly pull out their Worlds Best Federal Inspector of Medicine mug that they fill with their thermos with the Pfizer and AstraZeneca logo on it in the SHAPE OF A PILL, didn't just suck their will to live, he most certainly told them that every single cancer patient that can get the current SOC, will get SOC.
There is 1 silver lining element here that I would love to actually see datamined out (yes this is what datamining is); patients were not screened for fully functioning immune systems, meaning there were patients that could have been removed from the trial that were not as the drug would have had no way to cause a significant clinical effect. And that is where the real question begins with the Cisplatin + Multikine data. Was it because Cisplatin destroyed the immune system, thus stunting any long term immune response from Multikine, or is Multikine and Cisplatin not compatible on a more complex mechanistic teeter?
youtu.be
This is where I suggest CVM already knew Multikine wasn't going to work with Cisplatin. The way the trial was set up from the beginning illustrates they knew Cisplatin was never going to play nice.
I would like to point to Keytruda's first phase 3 results, and their FDA clearance history:
www.ncbi.nlm.nih.gov
Hint: Keytruda doesn't cure anyone. It just gives you more time, which is absolutely incredible and should not be taken from anyone. But it isn't a cure, it is a part of the cure .
www.drugs.com
June 30, 2014 - Keytruda is under regulatory review
September 4, 2014 - Keytruda is approved by the FDA
Keytruda gave people a few more months to live and it did it safely, without severe adverse effects. Keytruda was approved in 2 months.
Dispelling some of the fake newslines
The major reason this has taken over 30 years: THERE IS NO PRECLINICAL TEST TO TELL IF THIS THERAPY WORKS ASIDE FROM NON HUMAN PRIMAPES
It is invariably going to be tag line for short hedgefunds to stress "if it is so good, then why did it take 30+ years to get here". Unfortunately, the answer is the system. The system sucks, and the system has led to countless deaths because they are incredibly corrupt, inept and archaic. I am gifted by an interesting career in biotechs. I also have an interesting history in the financial markets. Part of this gives me insight into the crippling corruption in the CRO system, all the way to the approval system of the FDA, where countless lawsuits have proven correct that, yes, the FDA does accept bribes. CRO's are corrupt and disgusting monster corporations, anyone interested in a storied one could look no further than Parexel, which was getting bogged down with so many lawsuits and allegations that they had been abusing their position and purposefully not enrolling patients in small-scale biotech's clinical trials so their drugs never got through the long trials. There has been a number of interesting academic papers that have come out looking at the success/failure rate of drugs through clinical trials, and how prior to ~2012 something like 99% of drugs came out of large scale pharmaceutical companies. Unfortunately, as these papers are academic and the authors lacks a fundamental understanding and history of the system governing the clinical trial system, they fail to point out how many of these large scale pharmaceutical companies bribe CRO's, bribe the FDA, and work to spin extremely negative news in these clinical trials. Literally, these scumbags have gotten a hold of clinical study trial participant lists and called them all and fucking with the system by making them think they were going to die if they got the drug.
Want a sample study, look not further than our very own CVM: www.businesswire.com
There are no pre-clinical assays, especially not 30 years ago, that could give you the effects of a working immune system on a tumour. Mice do not have working immune systems, nor is there a guarantee any of the components in Multikine retain their appropriate pathway responses in any other species aside from humans. The major issue with a lot of diseases, like Alzheimer's, is the lack of a suitable test for drugs to know if they have an effect or not. It is not practical to screen drugs in patients (also not ethical), but the system has made it even more difficult to take drugs that work in human specific pre-clinical assays but do not have a suitable animal model to get into clinical trials. This has gotten better, but only very, very recently as the FDA has been slowly getting changed by a new wave of scientists and more-forward thinking government employees. Part of that leads us to the latest fiasco where an Alzheimer's drug without a clinical effect gets approved and cost ~$60k per year, which no way any nationally subsidized health insurance is going to pay. Part of that leads us to the countless fiascos of the countless drugs that have fallen through the cracks due to the system.
Fortunately, here we are, in 2021, with clinical trial results in hand and a much less suitable drug given the royal treatment as a role model.
cel-sci.com -page 6 for Head and neck cancer reasoning -pg 8 also good read
www.bloomberg.com -good discussion of Keytruda in this shareholders letter
Summary of Phase 3 FDA Trial
Multikine cures cancer in a specific subset of Head and Neck patients who did not get Cisplatin, which destroys the immune system, thus negating the effects of the drug.
Multikine had no negative medical events, meaning it was completely safe for EVERY SINGLE PATIENT WHO GOT IT.
Multikine activates the immune system against the tumour, similar to the way a vaccine allows your immune system to hunt down a virus or bacteria extra-well, but in a much more advanced way.
Theoretical Medical Relevancy
CVM's Multikine gets the immune system going red hot against solid tumours, but there is no study or data on how it would behave against a metastasized secondary tumour site, or against a metastatic cancer in general. I would not be surprised if Multikine is used in every single cancer given the patient has a working immune system. Furthermore, if Multikine is found to be safe in repeating doses, I can see maintenance Multikine being a serious clinical strategy.
Furthermore, CVM has preclinical studies going on Multikine in COVID infection, likely investigating more chronic viral infections as well. If Multikine is able to blanket activate the immune system, causing it to be super thorough, there is no reason not to consider it in any and every single infection scenario, viral and bacterial. I absolutely can see Guillain-Barre syndrome in a much broader population study, or other various auto-immune system issues, but part of me wants to say that this can be removed with dosages, and part of me is curious if the multi-pronged interleukin pathway activation from Multikine doesn't offer some sort of critical auto-immune system inhibitor. This is a completely novel therapy, and the immune system *typically* tries not to destroy its host. An interesting read on guillain-barre here (pubmed.ncbi.nlm.nih.gov) shows that some anti-interleukin 17 agents are possible treatments for this, meaning if negative side effects do happen, the fact that the alternative is cancer killing the patient, it is doubtful this hinders the drug, and that medically therapeutic options exist. Truthfully, most GBS events and auto-immune events are scary but transient. Interestingly, the interleukin pathway is crazy, and really still not fully known. Some of the interleukins inhibit each others pathways, some of these in very specific ways to modify the effects. This is just going to be one of the things that has to pop up in large scale clinical trials, especially as Multikine is tested in countless other therapies where the native immune system can be leveraged to do its job (i.e. every single infection).
Multikine getting FDA approval is absolute, to what scope is less so. Multikine will get open label use for cancer, but whether any research hospital chooses to bend that for study in other biologically relevant illnesses is another. With the Immune system being the flavour of the decade, I can only imagine how deep clinicians reach in for this one. Theoretically, Multikine would be effective in wounds, especially gunshot/knife wounds where there is a clear point of entry for infection. Multikine could be used as an emergency treatment used as an adjuvant therapy while the patient is stabilizing and wound closed. I am scientifically and medically excited for the uses of this drug.
Personally, I would love to see liver cancer treatment with Multikine, specifically liver because liver exosomes are particularly easy to purify from Urine, meaning you could monitor biomarkers routinely through the treatment effect, given they were present in urine exosomes of course. It is a little unethical and cruel to do, but getting some tumour samples would be amazing during the treatment as well. Ideally, Multikine is activating the immune system in the tumour region where the immune system is gathering epitopes against the mutated proteins on the tumour, or to simplify it a little, the tumour starts looking foreign to the immune system as it makes errors here and there and "new" proteins. These are hypothesized to be present and if the immune system could be activated against these, they would be able to hunt down the tumour cells at the primary site, but would be primed against any re-establishment of the tumour should a cancer stem cell survive or a metastasized colony is present elsewhere, the immune system should now be able to hunt them down and kill them everywhere. It would be very cool if we could take the immune system population from a patient treated with Multikine, and see if they are specifically targeting that patient's tumour versus other primary tumour samples.
Thesis - Price Action/Manipulation Strategy
thc-lab.net
This article is absolutely worth while in reading. I am sorry that this is the way the world works, I truly am. I believe that we are close to real change, but I think it's more thanks to Bitcoin than anyone wants to admit.
Looking at the short ratio reported day in, June 28th the short volume is monstrous, the way it is 50-50 makes me think this is a bunch of phantom shorts that were sold short before borrowed, with a hypothesis that the resurgent volume spike on the 30th might have been short hedgefunds recycling their shorts on the T+2 cycle. The fact they haven't yet from the June 30th intrigues me greatly, could be they found the way to bury them matched call:puts as there was a massive spike in far out of the money puts and options on the second half of the week. Alternatively, we could see a volume increase on a T+3 cycle, as is common in those looking to take the longest time to cover their previous short sales, willing to take a small fine if they get one on abusing the T+2 date, but still way ahead of the T+6 antics of Penson Financial(now Apex), and still even way more ahead of the T+21/T+35 FTD cycles that we can see repeating in the volume spikes (and congruent price spikes) over the past few months since the initial impulse to 40. Either way, should be a fun week.
www.nakedshortreport.com
Thesis- Price Target and Discovery
Fact, M&A's are red hot right now. Also Fact, the markets are really wonky right now. There is no guarantee on a blockbuster acquisition being possible even if the drug really is all that, just because the banks don't know how to stop being greedy. However, you cannot take the value out of a drug that cures cancer, so while an M&A could take a while to precipitate, the price action should reflect the massive increase in value of CVM, especially given the way the market has been valuing M&A targets previously such as QuantumScape, Tilray/Aphria, and countless others (wow the M&A market really has been red hot).
I have hit some intrinsic form of word limit, so I will keep it short. I see a short term market capitalization anywhere from the $1.5-3 billion range, where we could see extreme impulse ranges in the $10-30 billion market capitalization (the short squeeze trend has made these massive volatility channels outrageously hard to predict but fantastically enjoyable as a long). Truthfully, I could see an M&A deal panning out extremely quickly in the $5-10 billion range should a company rush quick while CVM is still learning to count 0's, but I would rather them take the time to get the full value where we could see the biotech records being broken, because again, this drug cures cancers.
TA is nuts to do right now just because the rules got changed so hard, and the way the price is so obviously being manipulated with the rhythmic ladder attacks and buybacks and the insane drops timed with margin shuffles. I wouldn't try and read the lines too much here, looking to hop in and out of channels as they come and go. Set a price target, set a loss limit, always do your research.
Check out pharmaintelligence.informa.com
Disclaimer
Thank you for reading this analysis. The sole purpose of these are to serve as an educational resource for any one to gain an understanding of what to research when deciding to make an investment. If there is any material in this analysis that you feel could be explained better, or in more detail, or even if you have just a little question, let me know! As I develop this skill, my aim is to expand the reach of these articles to cover more topics to a wider audience. Feedback helps me grow, so I am happy to read the comments.
For legal and ethical reasons, this is not financial advice, I really really really cannot stress that enough. Furthermore, I do own 4200 shares at an average price of $14 per share. This information is given due to European financial regulations, not as financial guidance. This information is only accurate as of the date of publication, July 6th, 2021. The original, and only version published by me is on Tradingview.com.
SENS Back Testing The 382 Fib Watching To Act As SupportThe thing about SENS is that it's been a company crushing it in 2021. When it was first discussed this year it was still trading below $1. Despite recent volatility, it's up significantly year-to-date. I think volume remains steady but definitely on the lower end of the range. But good data from the last presentation on its PROMISE study seems to have sparked more attention.
"The data evaluating the safety and accuracy of the next generation Eversense system was presented by Satish Garg , MD, Professor of Medicine at the Barbara Davis Center of the University of Colorado, Denver , and the PROMISE study group Principal Investigator (PI). The Company presented previously released information demonstrating performance matching that of the current 90-day sensor available in the United States , with reduced calibration, down to one per day, with duration extended to 180 days. Accuracy measurements discussed in the oral presentation include a mean absolute relative difference (MARD) of 9.1% for the primary sensor and confirmed hypoglycemia alert detection rates at 60 mg/dL of 87% and at 70 mg/dL of 93%. For the subset of 43 modified sensors (referred to as the SBA sensor), the MARD was 8.5% and the confirmed hypo alert detection rates at 60 mg/dL and 70 mg/dL were 90% and 94%, respectively."
Another area of potential support/resistance is just below the 382 fib line (in yellow), which has held so far this week. It was resistance a few weeks back. With SENS failing almost right at the 236 Fib line, technical traders are liking monitoring these levels closely.
Latest on SENS: Best Penny Stocks to Buy Right Now? 7 Small-Caps For Your Morning List