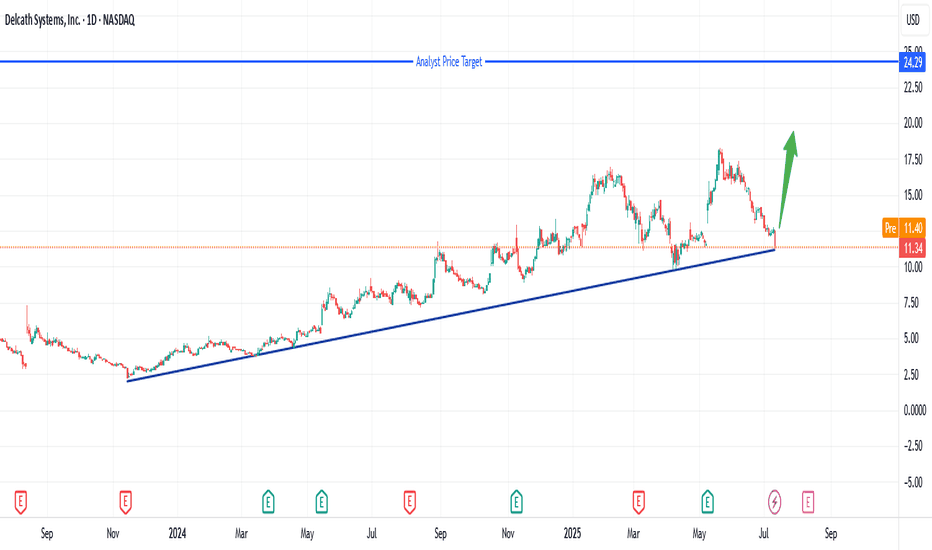
Explosive Rebound: Delcath at Critical Support Zone!Delcath Systems (DCTH) has pulled back to a key long-term support trendline after a sharp correction, presenting an exciting opportunity for a powerful rebound. The stock has respected this rising support for over a year, and buyers have historically stepped in at this level.
With the analyst price target set much higher at $24.29, risk/reward here is highly attractive. A confirmed bounce could ignite strong momentum toward previous highs and beyond. I’m watching closely for bullish price action and increased volume to signal entry. Stop placement should be just below the trendline to manage risk.
Not financial advice—always use proper risk management and do your own research!
DCTH trade ideas
DCTH Trade Setup – Key Levels! 📊
SL (Stop-Loss): $15.18 🔴
Entry: $16.13 🟡
T1 (Target 1): $17.19 🟢
T2 (Target 2): $18.28 🟢
👉 Monitor for confirmation of the breakout and manage risk wisely. 💹
#DCTH #StockTrading #TechnicalAnalysis #BreakoutTrade #SupportAndResistance #TradingStrategy #StockMarket #SwingTrading #ChartPatterns #DayTrading #RiskManagement #MarketAnalysis
$DCTH Banking On Dilutive MetastasisAlthough FDA approval is a promising sign to invest in biotech stocks, that may not be the case with Delcath Systems, Inc. (NASDAQ: DCTH) whose treatment for a form of metastatic melanoma – Heozato Kit – was recently approved by the FDA. The promising biotech company is suffering from high cash burn and its current cash balance is insufficient to last it more than 2 quarters at its current burn rate. With this in mind, the company’s expenses are set to drastically increase since it intends to make its treatment available for commercial use in Q4. Considering that the stock is up 82% since receiving FDA approval, shorting DCTH stock may prove profitable since capital raise may be announced soon.
DCTH Fundamentals
At times, dilution is the only means for a company’s survival such is the case for DCTH. The company had a cash balance of only $14.5 million and an operating loss of $8 million in Q2 2023 and due to its operating costs, the company burned through $13.9 million in the first half of the year. Having said that, this is merely the tip of the company’s cash burn iceberg due to the fact that it recently received FDA approval for Hepzato Kit, which means that it is expected to start mass production in order to increase its product revenue.
Since the company expects to introduce its treatment to the market in Q4, it is fair to assume that mass production will start this quarter which means that the company’s operating costs will drastically increase. For reference, operating costs are high compared to its cash balance as they currently account for $8.3 million, and with production expected to start soon, this figure may reach another order of magnitude. Taking that into consideration, the company may not be able to start production without a capital raise given its current cash balance.
Thankfully, the FDA approval triggers the second tranche of the company’s financing regulations which will initiate a private investment in public equity (PIPE), during which, PIPE investors have 21 days to exercise their tranche A warrants in exchange for $34.9 million in funding. Although this funding is critical for the company, it comes at the cost of diluting shareholders. Moreover, since the company is already burning around $8 million per quarter pre-production, these funds would only last for a year without considering the added costs of mass-producing its treatment.
Once the company starts production, its expenses will substantially increase as well as its cost of revenue which is why it may seek out a public offering to capitalize on the stock’s high PPS following its run on FDA approval news which would further dilute shareholders. Moreover, if the company generates $10 million in US sales from its new treatment, PIPE investors would have 21 days to exercise their B warrants – providing the company with an additional $24.9 million. Given that dilution appears to be a certain fact for the company, DCTH stock may fall soon which makes shorting it at current levels a potentially profitable decision.
Technical Analysis
DCTH stock was trading in a bearish trend as it was in a downward channel. However, the stock broke this channel after receiving FDA approval – creating a gap near $3.14 in the process. Looking at the indicators, the stock is above the 200, 50, and 21 MAs which is a bullish sign. Meanwhile, the RSI is approaching overbought at 65 and the MACD is approaching a bearish crossover.
As for the fundamentals, DCTH stock may be poised to fall if the company announces a capital raise given its dwindling cash balance and its high cash burn rate. In addition, PIPE investors could now exercise their warrants which may add selling pressure on the stock price. Based on this, investors could go short on the stock with an entry on the break of support and take profits on retests of the 200 MA and the upper trendline, with a stop loss at $5.8 if it holds support.
DCTH Forecast
Despite receiving FDA approval, DCTH stock may be poised to fall drastically from current levels as the company may resort to dilution to raise the funds required to start producing its Hepzato Kit. Aside from this factor, the company has to dilute since PIPE investors are now able to exercise their warrants – providing the company with $34.9 million in dilutive funding. With PIPE investors also set to exercise their B warrants once the company achieves $10 million in US sales from its treatment and the possibility of announcing a public offering soon, going short on DCTH stock may prove to be a profitable decision.
DCTH REKT Alot Of PeopleHello,
What is DCTH?
Delcath Systems, Inc. Delcath Systems, Inc. (NASDAQ: DCTH) is a publicly traded specialty pharmaceutical and medical device company that develops percutaneous perfusion technologies for the targeted administration of high-dose chemotherapeutic agents to specific organs or regions of the body.
News
I have found that this company has had two reverse splits; Dec 24, 2019 1:700 Stock Split, Oct 22, 2019 1:100 Stock Split. Reverse stock splits boost a company's share price. A higher share price is usually good, but the increase that comes from a reverse split is mostly an accounting trick. ... Whatever value it has is just distributed over fewer shares of stock, thus increasing the price. Anytime a company does a reverse split, it is a signal that a company is in distress.
My Thoughts For You
The Acronym of this company brings to mind what it could mean to those who invested. "Death Comes To Hodlers" I have read many comments about this company and they are not good. Check out the comments here.
This stock could bounce up as a fake out but history does repeat itself and so the story goes, I was and was no more.
Good Luck, Cheers!
Be sure to comment, follow, like, and check out my profile for more trade ideas!
DCTH: Another GAP Up Play in the WorksNASDAQ:DCTH
DCTH is a wild lil' Penny Stock that is already gapping up in pre-Market. This company is really trying to rally back up over $1, and has seen a bunch of strong plays since posting its strong earnings report.
Although I'm a bit skeptical for any long-term plays with this, I'm expecting another 'pumped up' morning run with this stock today. Also.... Since the pump will likely be driven by large players, I don't expect it to respect the normal support/resistance levels. So, trade wisely!
Disclaimer: My analysis and opinions are mostly based on the current trends and chart analysis. I'm not an expert, but I am consistently profitable. That said, you are inevitably responsible for your own decisions. Play at YOUR own risk. Cheers.
DCTH Bullish because of ascending triangleHello everyone
Here is my analysis on the DCTH stock. For me it looks very bullish because of the ascending triangle. As I think we will reach new All time highs soon!
Dont forget all the news, and the thing that people say they maybe get delisted. I don't think so, I think their drug will get FDA approved.
Please do also your own DD.
Thanks :)
$DCTH Mid Term Goals could put this over $1+ In Weeks - Repost -Reposting as it didn't post properly the first time
$DCTH Highlights for the first quarter of 2017 and the recent weeks include:
First quarter 2017 revenue of $0.74 million, an increase of 100% compared with revenue of $0.37 million in prior year quarter.
CHEMOSAT treatment milestone set by SPIRE Southampton Hospital in the U.K. with more than 100 CHEMOSAT treatments performed, including eight treatments on a single patient.
Announced a Special Protocol Assessment (SPA) agreement with the U.S. Food and Drug Administration (FDA) for the design of a pivotal trial of Melphalan/HDS to treat patients with intrahepatic cholangiocarcinoma (ICC).
The American Journal of Clinical Oncology published a single-center retrospective review finding that the Company’s investigational percutaneous hepatic perfusion (PHP) with Melphalan/HDS offered promising results with a doubling of overall survival (OS), significantly longer progression-free survival (PFS) and hepatic progression-free survival (hPFS) compared with other targeted therapies.
Favorable data from two institutions were presented at the Regional Cancer Therapies Symposium and showed strong tumor response and overall survival with the Company’s investigational PHP therapy in patients with ocular melanoma that metastasized to the liver.
“During the first three months of 2017 we continued to advance our clinical development programs in ocular melanoma liver metastases and intrahepatic cholangiocarcinoma, while making steady progress with commercialization of CHEMOSAT in Europe,” said Jennifer K. Simpson, Ph.D., MSN, CRNP President and CEO of Delcath."
“As we announced recently, we have concluded a new SPA agreement with the FDA for the initiation of a pivotal trial for the use of Melphalan/HDS in patients with ICC. This new trial will enroll approximately 295 ICC patients at about 40 clinical sites in the U.S. and Europe, with the primary endpoint of overall survival and with secondary and exploratory endpoints that include safety, progression-free survival, objective response rate and quality-of-life measures. The trial is designed to be cost-effective and conducted in a financially prudent manner, with modest investment in this fiscal year. In conjunction with the FOCUS Trial in ocular melanoma liver metastases, our clinical development programs now include two paths toward potential U.S. market approvals.
“In Europe, we continue to make steady progress with the commercialization of CHEMOSAT. Our first quarter revenue of more than $0.7 million was double the prior year period’s sales, driven primarily by national reimbursement in Germany under the ZE system. With coverage under the ZE system now in place, we expect product sales growth from this market for the remainder of 2017."
"Elsewhere in Europe, we continue to focus on building the clinical and pharmacoeconomic data to support reimbursement applications in other key markets. We expect that positive negotiations for coverage in Germany will support our efforts for payment levels in other markets such as the U.K. and the Netherlands. Securing reimbursement coverage in additional European markets remains critical to future revenue growth for CHEMOSAT,” concluded Dr. Simpson.
As of March 31, 2017, Delcath had cash and cash equivalents of $6.4 million, compared with $4.4 million as of December 31, 2016. During the first quarter of 2017, the Company used $3.8 million of cash to fund operating activities. Delcath believes it has sufficient capital and access to committed capital to fund its operating activities through the end of 2017.
Also check out this article, pretty good read:
insiderfinancial.com